Abstract
Background:
Myeloproliferative leukemia virus oncogene (MPL)-mutated essential thrombocythemia (ET) is uncommon, with a cited incidence of less than 5%, while that of MPL-mutated primary myelofibrosis (PMF) is at least twice as frequent (Blood. 2006;108:3472;Blood.2008;112:141). MPL-mutated ET cohorts also have higher reported rates of fibrotic progression than their MPL wild type counterparts (Blood. 2014;124:2507). These observations suggest the possibility that some instances of MPL-mutated ET might actually represent prefibrotic PMF.
Methods:
Patients were recruited from institutional databases of the Mayo Clinic, Rochester, MN, USA. Diagnoses were according to the 2016 World Health Organization (WHO) criteria (Blood. 2016;127:2391). Study inclusion criteria required the availability of bone marrow biopsy slides for central review by one of the authors (C.A.H.). Central pathology review included assessment of bone marrow cellularity and extent of tri-lineage proliferation, megakaryocyte morphology and grading of reticulin fibrosis. Data was additionally collected from MPL-mutated patients with PMF, for comparison. Standard statistical methods were used for statistical analysis, including calculation of survival data (JMP® Pro 13.0.0, SAS Institute, Cary, NC, USA).
Results:
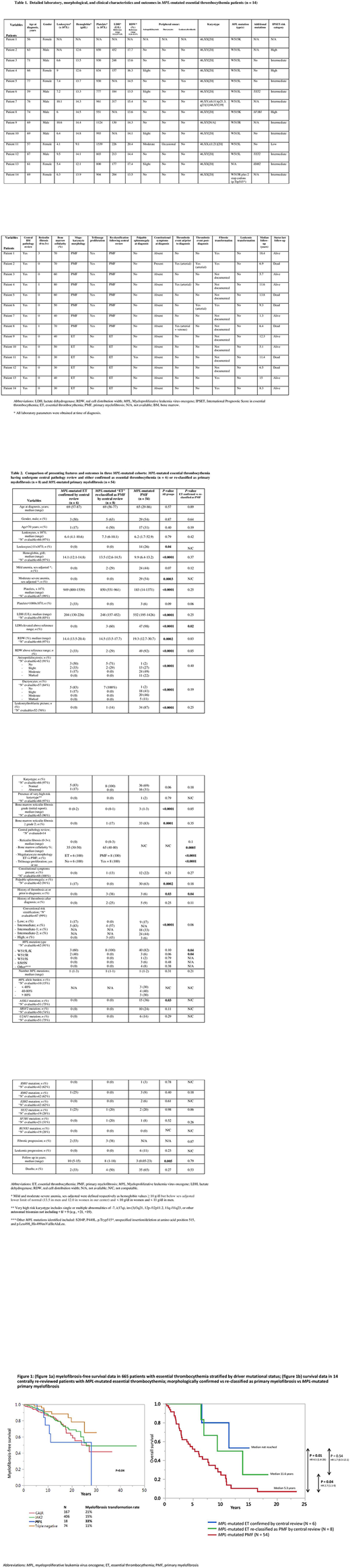
A total of 665 patients with ET were annotated for their driver mutational status; 18 (2.7%) were reported out as being MPL-mutated; by comparison, among 867 patients with PMF, 47 (5.4%) were signed out as MPL-mutated. Among the 18 cases with MPL-mutated ET, bone marrow slides were available for central pathology review in 14 patients (Table 1). The latter were subsequently reassigned the diagnosis of either prefibrotic PMF (n=8; 57%) or were felt to be morphologically consistent with true WHO-defined ET (n=6; 43%). Comparison of these two distinct histopathological patterns, i.e. true ET vs reassigned prefibrotic PMF, was respectively characterized by lower (median 35%, range 30-50) vs higher (median 65%, range 40-80) bone marrow cellularity (P<0.001), ET (n=6) vs PMF (n=8) consistent megakaryocyte morphology (P<0.001) and presence of tri-lineage proliferation (0% vs 100%; P<0.001); in contrast the degree of reticulin fibrosis was similar between the two (P=0.1) (Table 2).
The reassigned prefibrotic PMF (n=8), vs confirmed ET (n=6), cases also displayed a higher frequency of increased serum levels of lactate dehydrogenase (60% vs 0%; P=0.02), higher likelihood of displaying hemoglobin levels below the sex-adjusted reference range values (29% vs 0%; P=0.1), leukoerythroblastosis (14% vs 0%; P=0.2), constitutional symptoms (13% vs 0%; P=0.2) and a higher incidence of thrombosis history at presentation (38% vs 0%; P=0.04) (Table 2). Interestingly, reassigned prefibrotic PMF also displayed a narrower MPL mutational spectrum compared to those confirmed as ET (MPLW515L/K incidence 100% vs 60%; P=0.04). The incidences of abnormal karyotype and high risk molecular mutations (ASXL1, SRSF2 and U2AF1) were similar between the two.
We documented a higher incidence of post-diagnosis thrombosis in prefibrotic PMF (25% vs 0%; P=0.1) but similar rates of leukemic transformation (0% for both) and fibrotic progression (38% vs 33%); when all 665 ET patients were assessed for myelofibrosis-free survival, MPL-mutated cases (prior to central review) displayed significantly worse outcome, compared to patients with other driver mutations (Figure 1a). Median overall survival in confirmed MPL-mutated ET, ET re-classified as PMF, and MPL-mutated PMF was not reached, 11.6 and 5.3 years, respectively (confirmed ET vs PMF, P=0.01; ET re-classified vs PMF, P=0.04; confirmed ET vs ET re-classified as PMF, P=0.54) (Figure 1b).
Conclusions:
The current study suggests that the majority of routinely-assigned MPL-mutated ET probably represents prefibrotic PMF, when morphologically scrutinized. Prior to central pathology review, we show a higher rate of fibrotic progression in MPL-mutated ET, compared to patients with other driver mutations; accordingly, after central pathology review, the similar rate of fibrotic progression between morphologically confirmed MPL-mutated ET and those reassigned as prefibrotic PMF further suggests the latter to be biologically more akin to PMF, despite its ET-consistent morphologic features.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal